The outbreak of the Covid-19 pandemic, stagnant domestic consumption and weak external demand slowed Vietnam's economic growth last year, but growth momentum will continue to be strong this year and next, thanks to Vietnam's success in controlling the spread of the virus,” said ADB Country Director for Vietnam Andrew Jeffries. “But this year and next there are still significant risks, including the return of new coronavirus variants.”
By mid-June, despite such a high vaccination rate, the number of infected people still exceeded 10,000 cases/day. World oil prices and domestic consumption, together, are expected to push inflation to 3.8% this year and 4.0% in 2022.
So we see, in the context of the Corona virus causing great harm to both health and economy, Vietnamese vaccine manufacturers in general and NANOGEN BIOTECHNOLOGY JOINT STOCK COMPANY said Particularly, it has been, is and will continue to strive in the big but feasible ambition that: the Vietnamese vaccine will soon succeed and the Vietnamese people can protect themselves by self-reliance.
ABOUT NANOGEN BIOTECHNOLOGY JOINT STOCK COMPANY
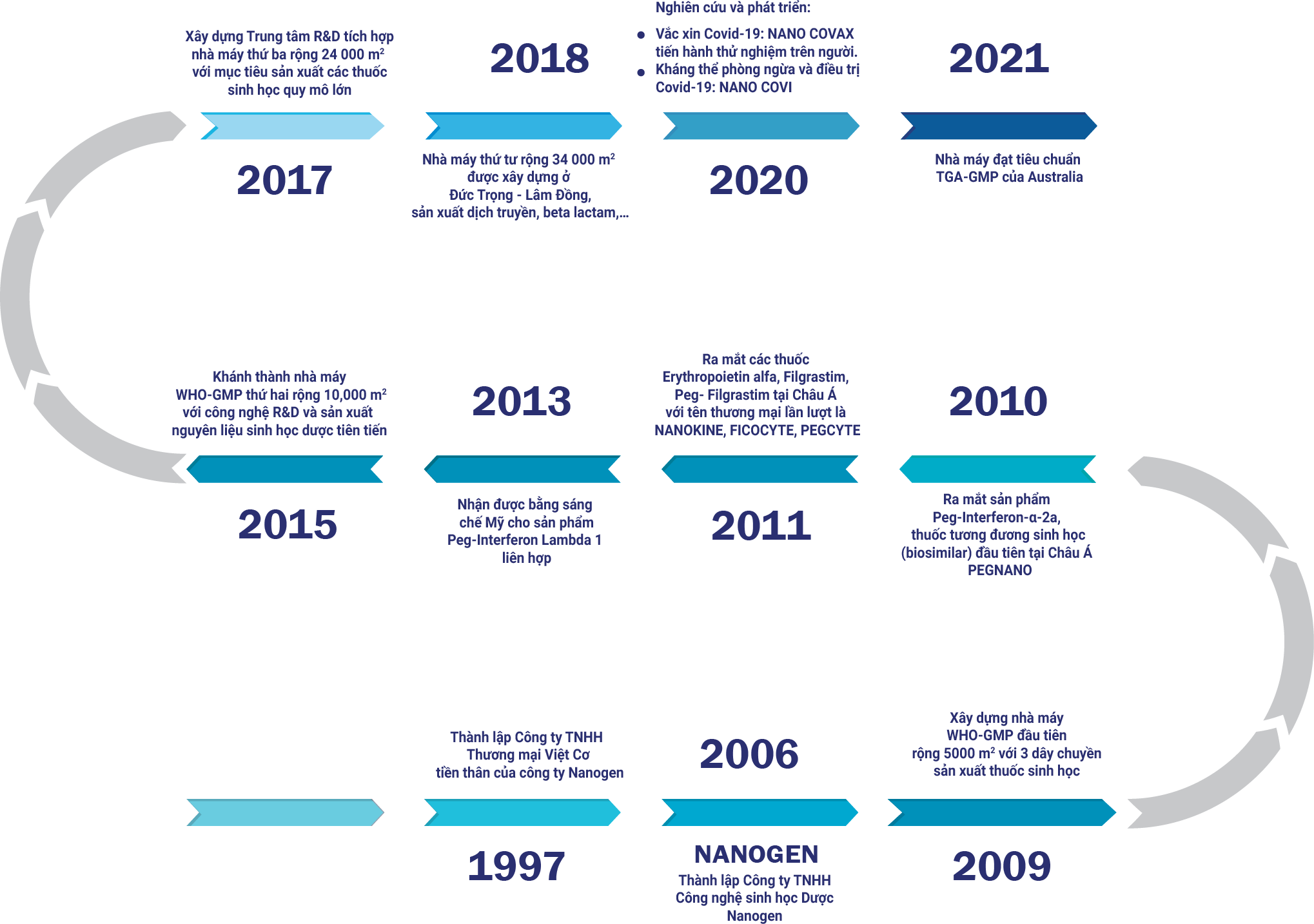
Established in 1997, Nanogen Company specializes in manufacturing pharmaceutical raw materials and finished products for special injections from recombinant DNA/protein technology.
Owning a line of special treatment products for hepatitis B, hepatitis C, anemia, cancer,.. In addition, Nanogen constantly researches and invented antibody product lines used in immunotherapy for cancer treatment. , vaccines and therapies to treat Covid-19, …
NANOCOVAX USES RECOMBINANT PROTEIN TECHNOLOGY TO CREATE NATURAL IMMUNE SYSTEM SAFELY AND EFFICIENTLY
Nanogen follows a different path than some other pharmaceutical companies are doing (implanting viral gene segments directly into people). Nanogen uses antigen-based vaccine production technology, using CHO cells - a type of cell with a design similar to human cells (exclusively by Nanogen) - as the medium to create the spike protein S.
Although the yield of spike protein S produced from CHO cells is small and the price of CHO cells is high, Nanogen still decided to use it because the spike protein S produced from CHO cells is of good quality (similar to real S spikes). Therefore, it will stimulate the body's high immune response to vaccination.
The spike protein S is then harvested, purified, and mixed with adjuvants to create the vaccine product. Because it does not use any live components of the virus, Nanocovax is safe and does not cause any serious side effects.
Dr. Ho Nhan - General Director of Nanogen Company - once said and affirmed that: "The way to make vaccines by direct gene implantation is faster, but the risk is much higher". Nanogen's goal is to create a vaccine that can protect the body 100%.
This is also the first time a private unit has stepped into vaccine research and production and has demonstrated its own advantages. That being said, it must be mentioned that Nanogen Company has both research capabilities, modern production lines and, importantly, a great spirit of determination.
Clinical Trial Phase 1: NANOCOVAX ACHIEVE 100% SAFETY
Since April 2020, Nanogen Company has embarked on researching drugs and vaccines against SARS-CoV-2 virus. By mid-December 2020, after successful research, production and mass testing on animals, the Ministry of Health officially pressed the button to allow the phase 1 trial of the Covid-19 vaccine produced by Nanogen on humans.
On February 8, 2021, Nanocovax completed 120 injections of phase 1 clinical trials in 60 volunteers. The research team's representative presented the results that: all 3 injection doses of 25mcg, 50mcg and 75mcg have 100% safety, 90% protection against SARS-CoV-2 virus infection and all All have good immunity.
Most of the volunteers are in stable health after injection. A few cases showed pain at the injection site, mild fever, but all disappeared after 1-2 days.
Clinical Trial Phase 2: NANOCOVAX's immunogenicity UP to 99.4%
On February 26, 2021, the phase 2 clinical trial of Nanocovax was started at the Medical Center of Ben Luc District, Long An, led by the Pasteur Institute. The second injection is given 28 days after the first injection. Over 560 volunteers, 18-65 years old and continuing to use all 3 dose groups.
As a result, the vaccine is safe, 100% of the volunteers are immune, the seroconversion rate reaches 99.4% and the antibodies produced can fight against the Wuhan virus and new strains. The side effects of Nanocovax are even lower than that of Pfizer and Moderna, no case of anaphylaxis has been recorded, all cases of fever are very mild from 37.5-38 degrees Celsius and go away on their own.
Specifically: Nanocovax 25 mcg, 50 mcg, 75 mcg all have Anti-S IgG content, neutralizing antibody activity, neutralizing antibody titres PRNT50 (the concentration of serum dilution can neutralize live virus). ) started to increase on day 35, and increased on day 42 after injection 1. The difference in immune response between these 3 doses was not statistically significant.
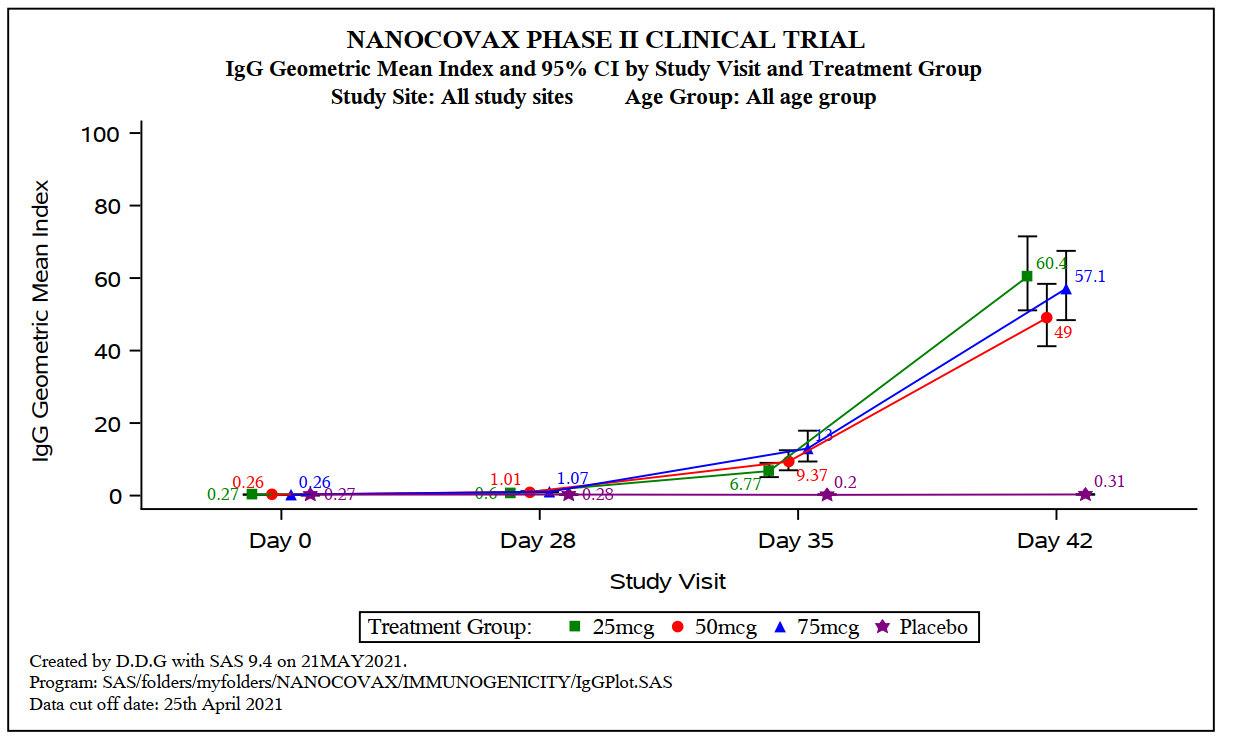
Biểu đồ:. Hàm lượng kháng thể Anti-S IgG
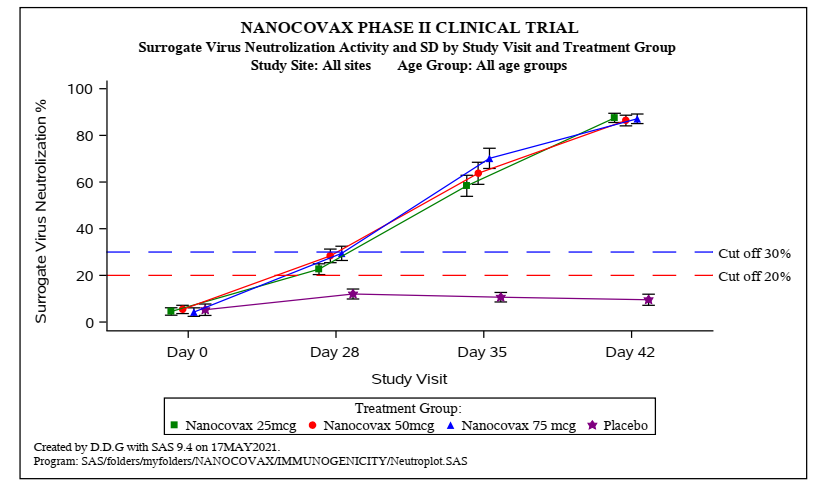
Biểu đồ: Kết quả Hoạt tính kháng thể trung hòa (Surrogate Virus Neutrolization)
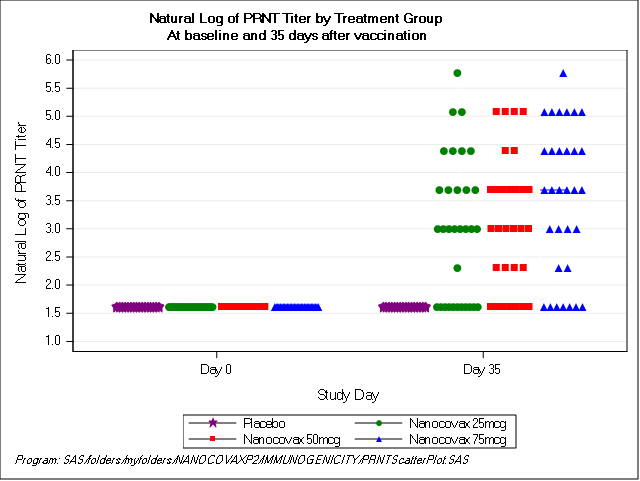
Biểu đồ: Hiệu giá kháng thể trung hoà PRNT
Clinical Trial Phase 3: HIGH PROTECTION IS POSSIBLE WITH NANOCOVAX
Based on the very good results of phase 1 and phase 2, at noon on June 11, 2021, the Ministry of Health officially approved the phase 3 trial protocol of Nanocovax vaccine (dose 25 mcg/mL) of Nanogen company. The above decision was signed by Deputy Health Minister Tran Van Thuan.
Phase 3 will be tested on 13,000 volunteers. And now, 1,000 volunteers have completed the 2nd injection. Naogen is trying to speed up the progress but ensure that there are no cuts or missed stages, ensuring the safety and high accuracy of phase 3. The company expects and hopes to complete this phase 3 clinical trial by September.
After completing phase 3 with good results, Nanogen will continue to verify the results of Nanocovax and then begin the process of applying for permission from the Ministry of Health and the Ethics Council to issue a conditional emergency license to allow circulation. Nanocovax.
The two main researchers in this phase were the Military Medical Academy in the North and Pasteur in the South. On June 15, 2021, Parteur started a trial injection at the Ben Luc site, specifically at the General Hospital of Ben Luc District, Long An Province. It is expected to start the phase 3 clinical trial of Nanocovax at the remaining 2 sites in My Tho and Cho Gao.
In the North, the Military Medical Academy will act as the focal point for deployment at Military Medical Hospital 103 and the Center for Preventive Medicine of Hung Yen province to deploy locally.
PRODUCTION CAPACITY
Nanogen has 4 factories, 3 factories in Ho Chi Minh City with an area of 5,000 m2, 10,000 m2, 24,000 m2, 1 factory in Lam Dong with an area of 34,000 m2 and 1 Research and Development Center. in Vietnam with a total area of 73 000 m2.
Based on the current plan and capacity, it is expected that by the end of 2021, Nanogen will be able to produce 50-100 million doses of the priority Nanocovax vaccine in the country.
And in order to ensure the source of vaccines for the community, Nanogen is trying to perfect and expand the cold storage system with a capacity of 10 million doses and a team of international standard refrigerated trucks (keeping the temperature 2-8 days). °C) to transport vaccines.
In addition, although Nanocovax is 100% safe and 99.4% immunogenic - not inferior to vaccines already circulating in the world - but thanks to the use of technology that the company has mastered. For more than 10 years now and with a good source of raw materials, the expected selling price of Nanocovax is currently 120,000 VND/dose (the lowest in the world).








