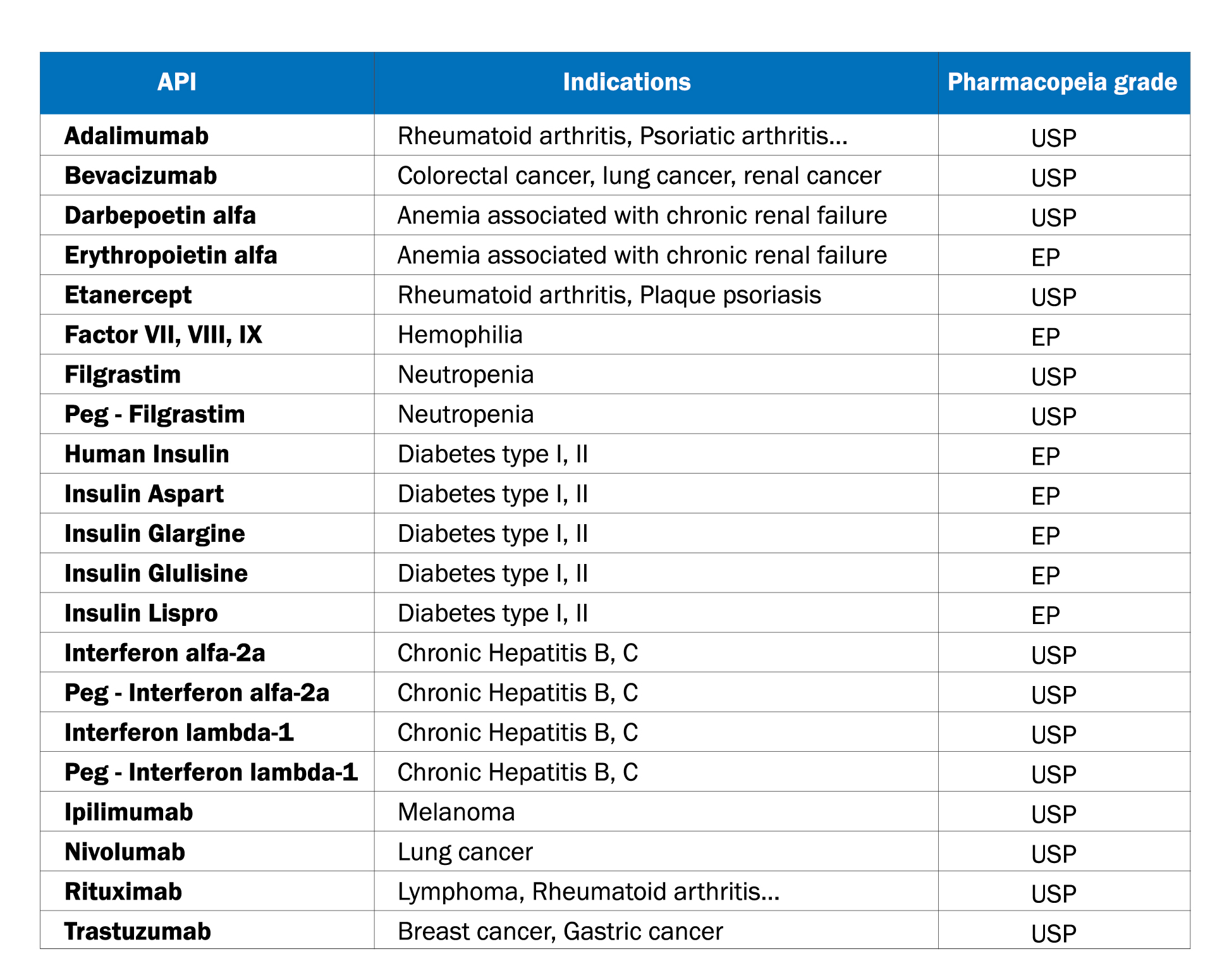
NANOGEN PHARMACEUTICAL BIOTECHNOLOGY JSC.
Address:
Lot E2a-1, E2a-2 Saigon Hitech Park, Long Thanh My Ward, Thu Duc City, Ho Chi Minh City, VIETNAM
Lot I-5C Saigon Hitech Park, Tang Nhon Phu A Ward, Thu Duc City, Ho Chi Minh City, VIETNAM
Tel: (+84) 28 7108 9688
Fax: (+84) 283 730 9963
Email: info@nanogenpharma.com