COVID-19 pandemic has become a global crisis that has seriously affected global healthcare systems and economics. Up to 20/11/2020, there were over 57 million cases and over 1 million deaths all over the world. In Vietnam, there were more than 1,300 cases, 35 deaths and some severe cases reported . Although the pandemic has been basically under the control in Vietnam, it could last for a long time with complicated developments in the coming period.
NANOGEN BIOPHARMACEUTICAL has more than 20 years of experience in researching and developing biopharmaceuticals on the foundation of recombinant DNA/protein technology. With the willingness to support the community in the fight against Covid-19, NANOGEN has been striving to invent SARS-CoV-2 vaccine and treatment by our advanced technology platform.
About SARS-CoV-2
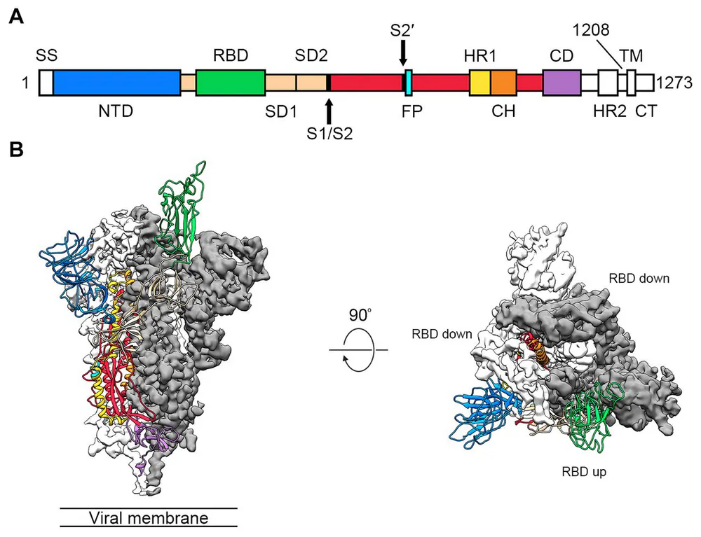
SARS-CoV-2 is the strain of coronavirus that causes coronavirus disease 2019 (COVID-19). Same as all other coronaviruses, the genome of SARS-CoV-2 (2019-nCoV) encodes the spike protein, the envelope protein, the membrane protein, and the nucleocapsid protein.
The spike protein (S-protein) is the common target for neutralizing antibodies and vaccines. Spike protein, a glycoprotein with the molecular weight of 141 kDa, is important in the infection process of CoV to host cells. Spike protein contains two subunits, S1 and S2. S1 contains a receptor binding domain (RBD), which is responsible for recognizing and binding to the cell surface receptor. S2 subunit contains other basic elements needed for the membrane fusion. SARS-CoV-2 (2019-nCoV) can infect the human respiratory cells by using this receptor to interact with human angiotensin-converting enzyme 2 (ACE2). The transformation of S-protein after interacting with the host cells helps the virus entry.
How does Nanogen SARS-CoV-2 vaccine work?
COVID-19 vaccines promote human immune responses to SARS-CoV-2 without us having to get the illness. With all types of vaccines, the body has “memory” T-lymphocytes as well as B-lymphocytes that will remember how to fight that virus when being infected in the future. Currently, there are three main types of COVID-19 vaccines that are in research and development: mRNA vaccines, protein subunit vaccines and vector vaccines.
NANOGEN developed and produced protein subunit vaccine by using recombinant S-protein subunit binding to silica nanoparticles. Rather than introducing the entire germ to the immune system, a subunit vaccine contains harmless antigen fragments (proteins) of SARS-CoV-2 and elicits an appropriate immune response.
In our sub-unit vaccine, the transmembrane domain of S-protein was removed. S-protein was produced by using recombinant DNA technology on CHO cells (Chinese hamster ovary cells).
S acted as a protein cargo carrying the S, M, E, N antigen proteins of SARS-COV-2 to stimulate the immune response. In order to increase the magnitude of the immune response, adjuvant was added to our SARS CoV-2 vaccine.
After more than 4 months of research, we successfully produced vaccine candidates based on S-protein (wild type and 4 mutant types). This vaccine was absorbed into aluminum particles with a specific adjuvant to stimulate the humoral and cellular immune responses. The initial step of cloning and screening stages was time-consuming but the most significant advantage of this kind of vaccine was high safety and high ability to stimulate the immune system.
The vaccine produced by NANOGEN Pharmaceutical Biotechnology (NANOCOVAX), would be the first Vietnamese Covid-19 vaccine to be tested on humans if approved, said Nguyen Ngo Quang, deputy head of the Administration of Science, Technology and Training under the Vietnamese Ministry of Health. The vaccine may enter human trials in November and it is expected to reach the market in 2021. Clinical trials would occur over three stages, during which the vaccine would be tested on 20 people, then 600, and finally over 10,000. The current manufactory system of NANOGEN is capable of manufacturing 20-30 million doses, up to 100 million doses each year to satisfy the domestic demand and export orientation.

Our new research direction in SARS-COV-2 treatments
We developed the Covid -19 treatments in two different orientations:
- Antibody fragment includes scFv and Fab against the receptor binding domain (RBD) of SARS-CoV-2 S protein expressed by E.coli. This antibody binds to and neutralizes the virus.
- ACE-2-Fc fusion protein is expressed by CHO. This protein binds to, neutralizes and stimulates the ADCC, CDC responses.
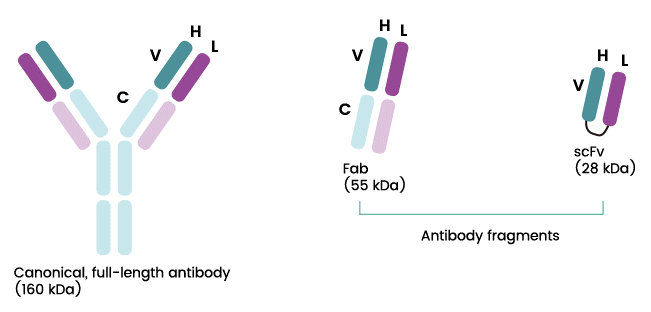
The scientists of NANOGEN had successfully conducted research on 4 types of scFv antibody based on 4 types of antibody oriented from recovered patients, which was proven to be capable of binding to the Receptor binding domain (RBD) of SARS-Cov-2 S-protein.
These kinds of antibody will be used as a cocktail and developed into different products: Prefilled syringe subcutaneous injection; nasal spray & inhaler; eye drop. NANOGEN had finished the research and development stage of scFv and peg-scFv to neutralize the activity of SARS-CoV-2 S-protein RBD. We expect to produce up to 10 000 doses per week and conduct trials in the coming period.
[1] https://www.worldometers.info/coronavirus/
[2] Huang, Y., Yang, C., Xu, Xf. et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin 41, 1141–1149 (2020). https://doi.org/10.1038/s41401-020-0485-4
[3] https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvaccines%2Fabout-vaccines%2Fhow-they-work.html
.png)
.jpg)
.jpg)




.jpg)


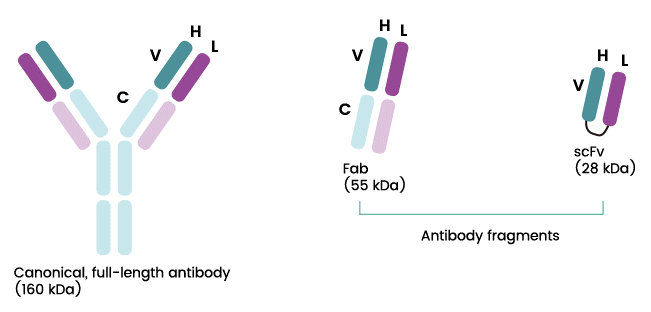
.png)
.jpg)
.jpg)
