NANOGEN PHARMACEUTICAL BIO TECHNOLOGY JSC. offer CDMO services to clients who need expertise and GMP manufacturing capabilities in pursuit of their clinical development or commercial objectives.

Nanogen is pleased to offer very competitive CDMO services to collaborators from cell line development to fill and finish, who wish to:
•Begin at the cell line development or process development stage and utilize Nanogen’s extensive expertise in biopharmaceutical development.
•Improve, optimise their product’s titre and/or scale-up an existing process to reduce the overall cost of goods,
•Transfer existing processes to Nanogen to utilise our GMP capabilities and capacities,
•Establish a manufacturing base in Vietnam or South-East Asia for their products.
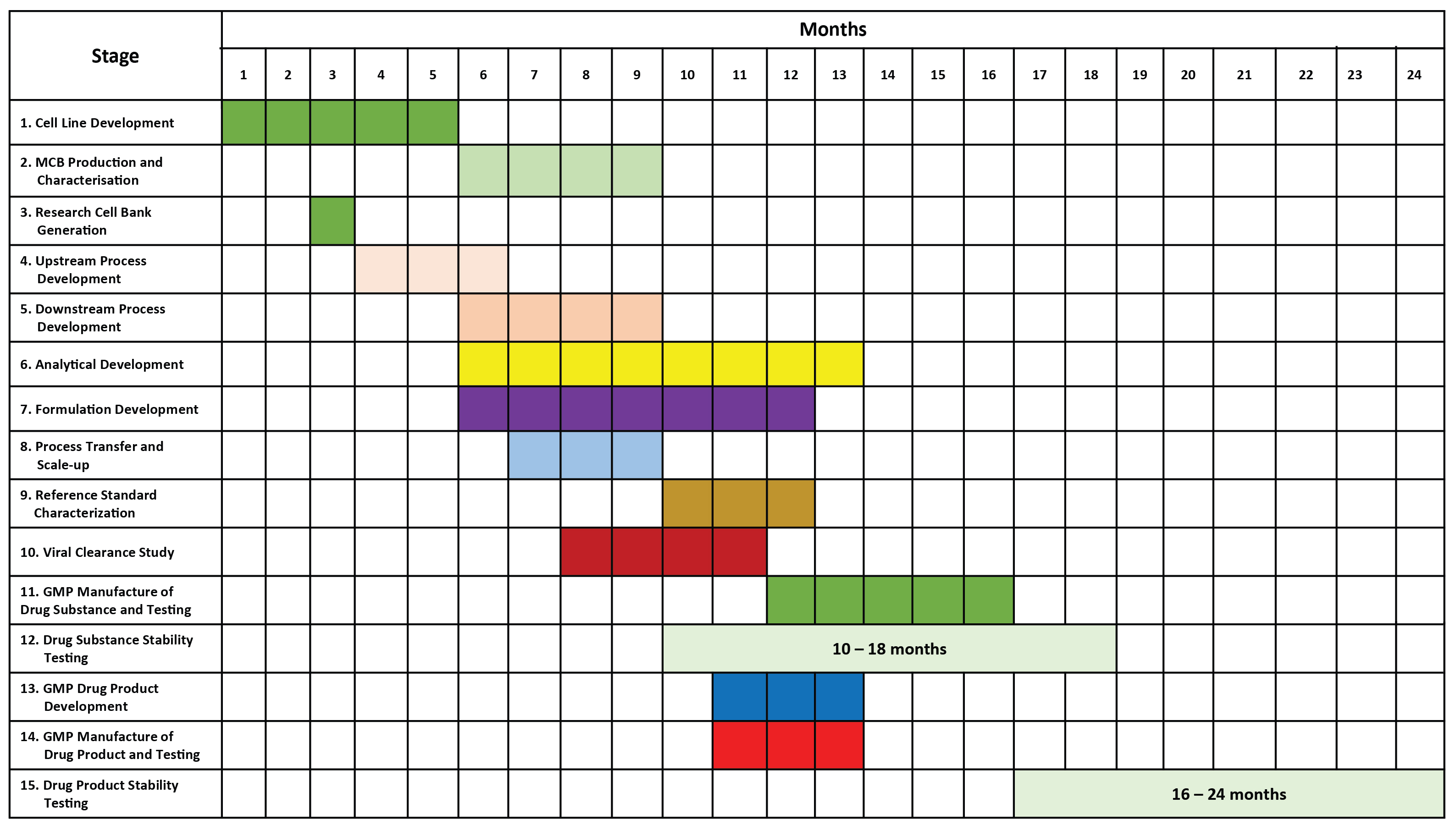
DEVELOPMENT TIMELINE
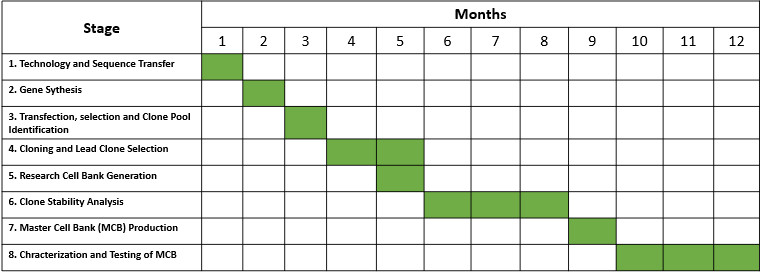
1. CELL LINE DEVELOPMENT
Nanogen’s scientists have extensive experience in mammalian (CHO) and bacterial (E. coli) cell line development. Nanogen has developed various cell banks for a number for monoclonal antibodies (biosimilars), recombinant proteins, hormones and recombinant vaccines.
• Transfection technology: Both CHO cell and E. coli,
• Process for cell line development: Transfection -> Selection of stable cell pools -> Isolation of monoclonal cell population -> Selection of best clone,
• Analytical methods for clone selection: Microscopy, FACS, ELISA, WB, HPLC,
• Deliverables: Research Cell Bank (RCB), CLD Development Report.
2. MASTER CELL BANK PRODUCTION
Examples of products we have developed:
• CHO cells:
• MABs: Trastuzumab, Bevacizumab, Rituximab, Adalimumab, Omalizumab, Ipilimumab, Nivolumab, Pembrolizumab and CB6.
• Other recombinant proteins: DEPO, EPO, F8, F9, Etanercept, Tenecteplase and
• Vaccine: SARS-CoV-2 Spike Protein.
• E. coli:
• GCSF, Insulin Human, Insulin Glargin, Interferon alpha-2a/2b/lambda/gamma and scFv.
• Peg-GCSF and Peg-Interferon alpha-2a/lambda.
Titres of protein produced: ~4 g/L for Mabs, 0.5 g/L for other proteins, 3-5 g/L for E. coli products
Deliverables: Vials of MCB, MCB CoA and Report.
3. UPSTREAM PROCESSING
Nanogen’s Upstream Development Team has successfully scaled-up and improved processes from 50L to 200L to 1000L using the Sartorius STR single-use technology platform
• Upstream Process Development: test different media and feeds/supplements to improve protein titer and protein quality (glycosylation).
• Ambr system (15mL and 250mL) for optimization of process: CHO cell and E. coli.
• Different bioreactor options for scale-up: 50 L, 200 L, 500 L, 1000 L
• Timelines: 4 – 8 months for upstream process development for products from CHO cells
• Deliverables: USP Process ready to be transferred to GMP, USP material for DSP development, USP Development Report.
4. DOWNSTREAM PROCESSING
• Using our platform processes and equipment, our Downstream Development team can quickly optimise your protein’s purification strategy.
• Nanogen are well equipped to provide the necessary expertise in chromatography (affinity, ion-exchange, hydrophobic interaction), UF/DF, virus inactivation (low pH) and virus removal.
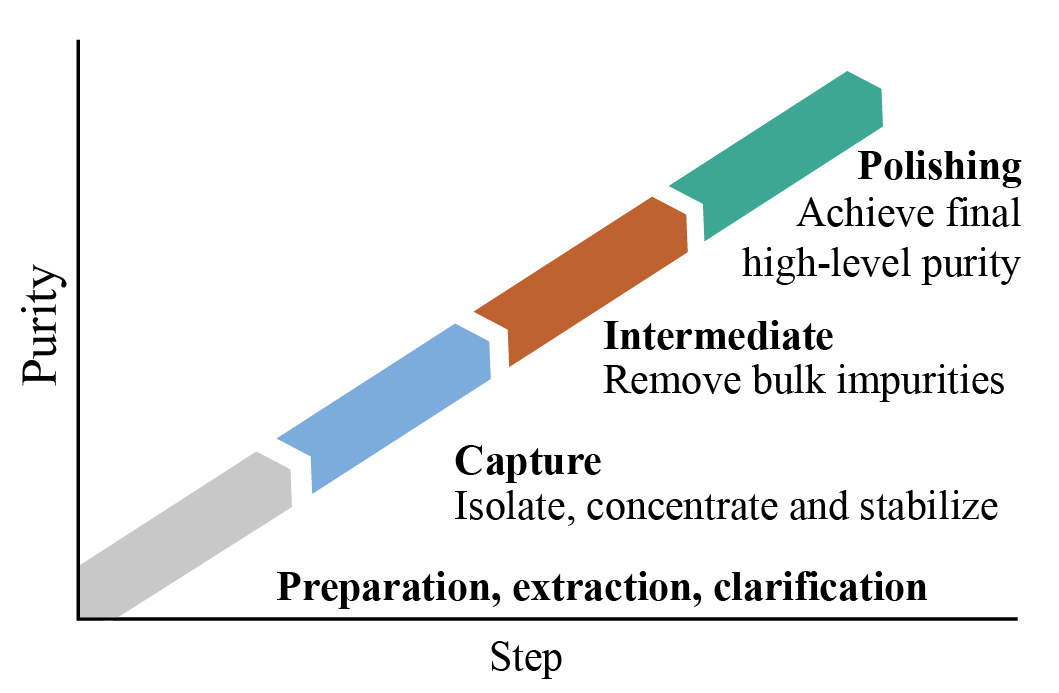
• Downstream Processing Strategies:
•Sample preparation: extraction, clarification.
•Capture: isolation and concentration of the target protein.
•Intermediate purification: removal of bulk contaminants.
•Polishing: removal of aggregates of the target protein.
• Different Methods of Chromatography: Affinity chromatography (AC), Ion exchange chromatography (IEX), hydrophobic interaction chromatography (HIC), etc.
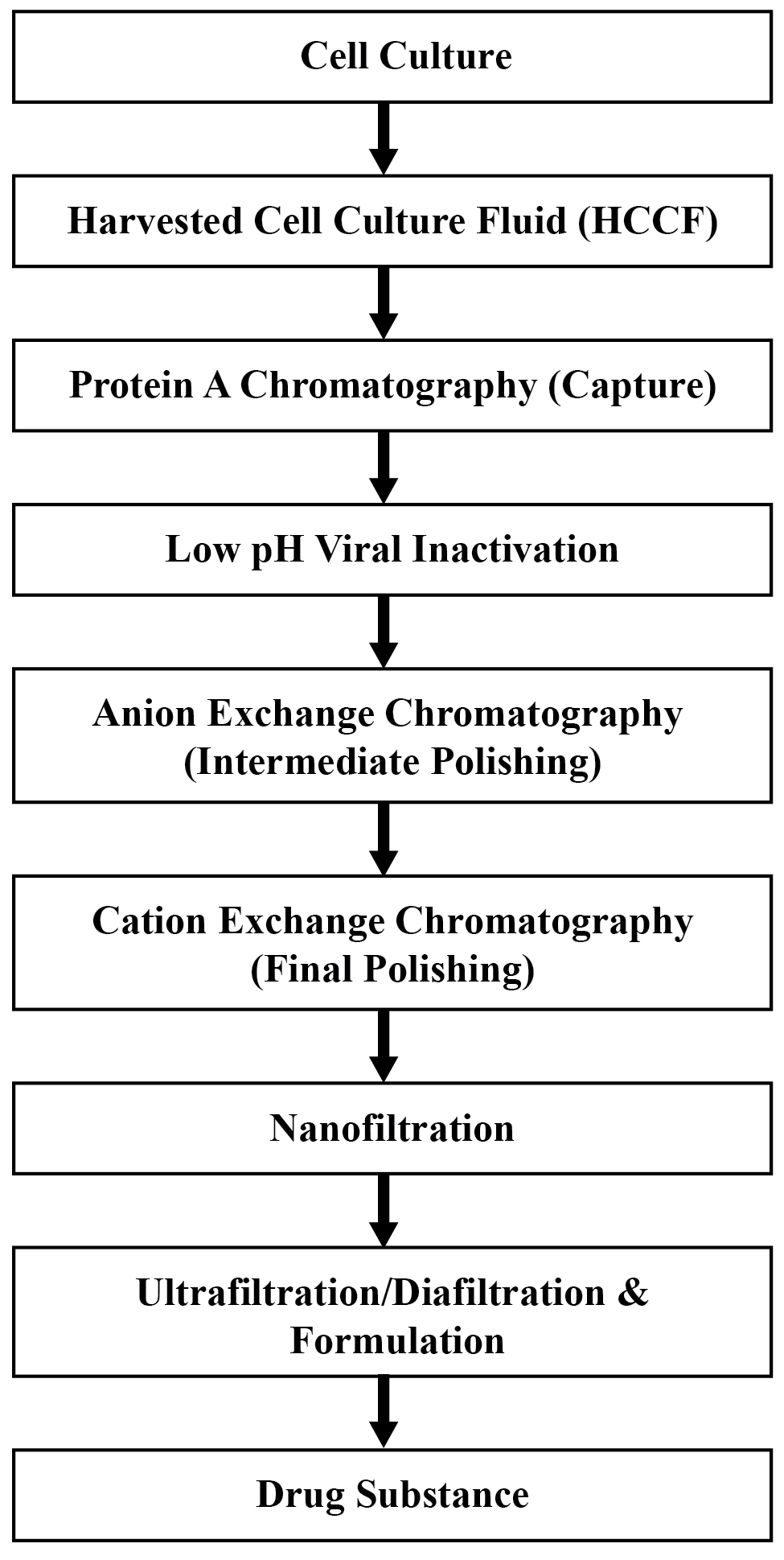
• Strategy for purifying Mab/recombinant protein: Based on capture, intermediate polishing and final polishing steps.
• Timelines: typically 5 days/ batch
• Deliverables: DSP process ready for transfer to GMP, material for viral clearance studies, formulation development, Tox pre-clinical studies, drug product development studies, DSP Development Report
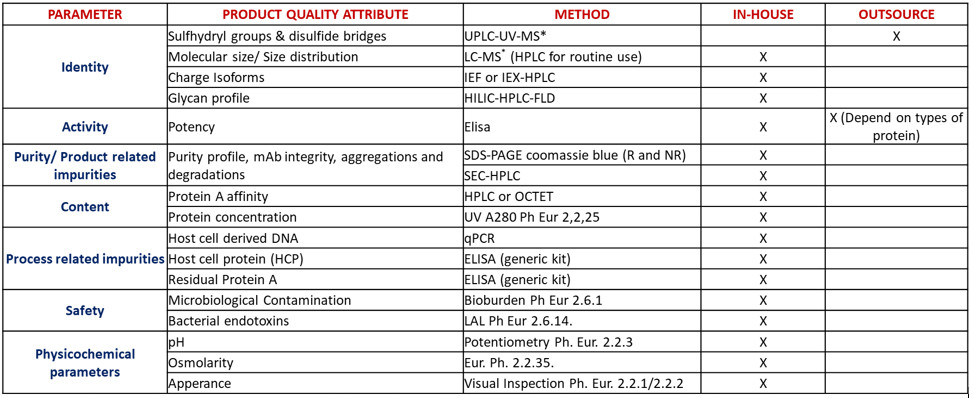
5. ANALYTICAL DEVELOPMENT
Our Analytical Development Team support our upstream and downstream development groups throughout the development process to ensure that the appropropriate test methods are developed to support specification development and a regulatory compliant panel of QC release test methods.
• Capable of carrying out a variety of analytical methods:
• In-house: HPLC, UPLC, capillary electrophoresis, Host cell DNA, Host cell protein, SDS-PAGE, Western Blot, animal potency test, etc.
• External partner: Protein sequencing, Viral clearance studies, etc.
• Validation of Analytical test methods.
• Transfer of Analytical methods using QA approved protocols and reports.
• Deliverables: Panel of analytical test methods used to characterise and release the drug substance, Analytical Development Reports, Analytical Qualification/Validation Protocols and Reports.
6. FORMULATION DEVELOPMENT
• Design strategy for formulation development: Mabs, Biosimilars and Vaccines. Evaluation of different buffers, pH, osmolality conditions, etc; and ingredients to stabilize the product: sugars, amino acids, detergents etc.
• Temperatures studied: 5±3oC, 25±2oC and 40±2oC.
• Analytical methods: SDS-PAGE, Western blot, HPLC, for evaluation of product stability.
• Deliverables: Preliminary data to support a lead drug substance formulation, Formulation Development Report.
7. DRUG PRODUCT MANUFACTURE
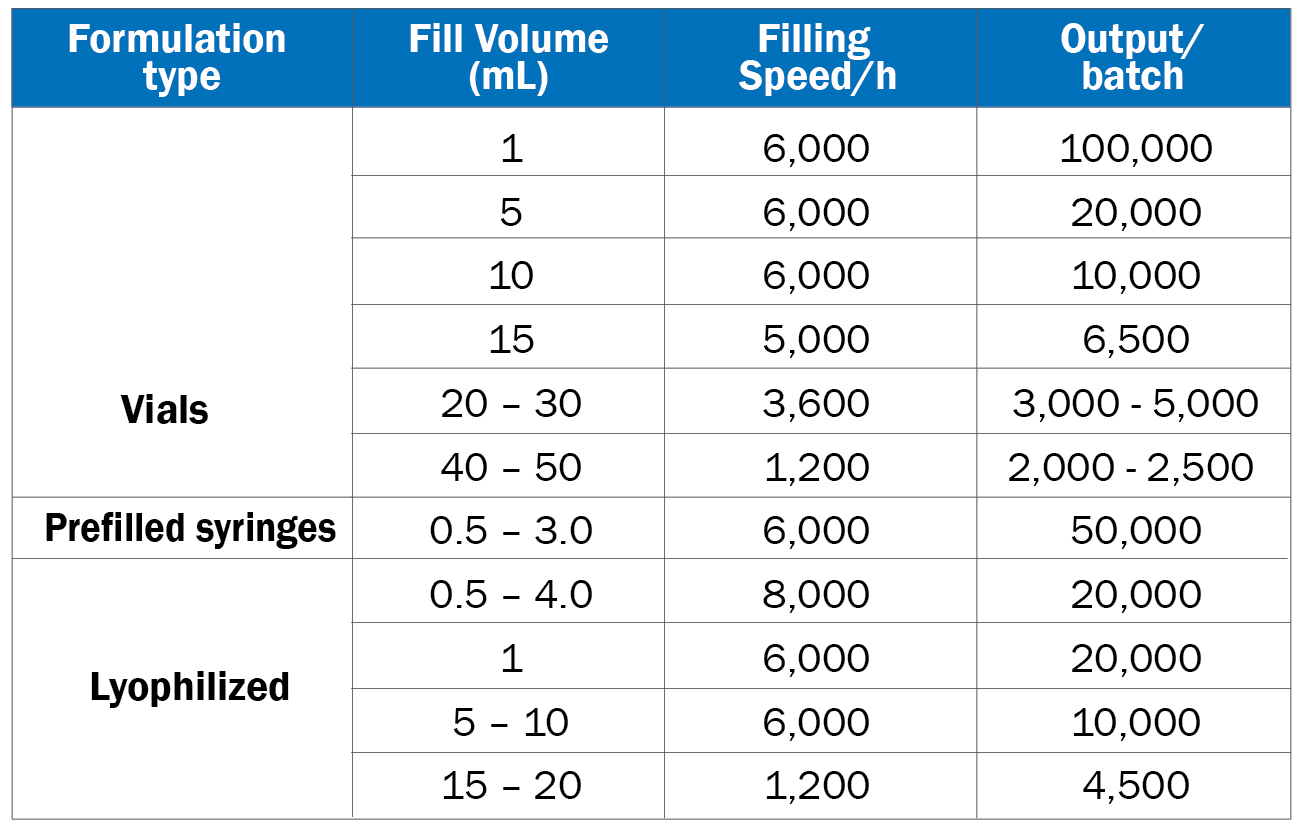
Nanogen has 3 fully automated (Grade A/ISO 5) filling lines which can offer clients liquid, lyophilised or pre-filled syringe options for their products.
• Production facilities: vial filling line, pre-filled syringe filling line, lyophylization system, refrigerated warehouse, etc.
• Equipment: Mixing tanks (200 L, 600 L), vial washing machine, vial capping machine, vial inspection system, etc.
• Productivity:
• Vial filling line: 120,000 vials per 24h (depending on vial size: 1 mL to 50 mL).
• Pre-filled syringe filling line: 40,000 syringes per 24h (1mL volume).
8. QUALITY CONTROL TESTING
Our Quality Control laboratories work closely with our Production teams to collect environmental monitoring samples and perform product release testing to support product disposition.
GMP COMPLIANCE
• Nanogen’s Quality Assurance group works to ensure that all activites are GMP compliant.
• Currently, Nanogen holds a GMP Manufacturing license from the World Health Organisation and Drug Administration of Vietnam.